Institutional Review Board
The purpose of the Institutional Review Board (IRB) is to review, approve, and monitor research involving human subjects and to protect the rights and welfare of human research participants.
Typically this includes:
- Protection from Physical and Psychological Harm: It is very important that participants will not in any way be harmed as a result of taking part in the study.
- Informed Consent Prior to Participation: Participants should be informed regarding who is conducting the study, its purpose, what participating in the study will involve, what risks are involved, and what the participant may gain from participating. Normally this includes a statement that participation is voluntary and participants may withdraw at any time. Those under 18 must also receive permission from a parent.
- Confidentiality: Data provided by participants will only be shared with those involved in the immediate research group and results will not identify anyone by name. Data is protected during the study and deleted or destroyed at the conclusion of the study.
- Deception and Debriefing: The IRB requires researchers to show that planned deception in the study will cause no harm. Participants in a study that involves deception must be debriefed afterward.
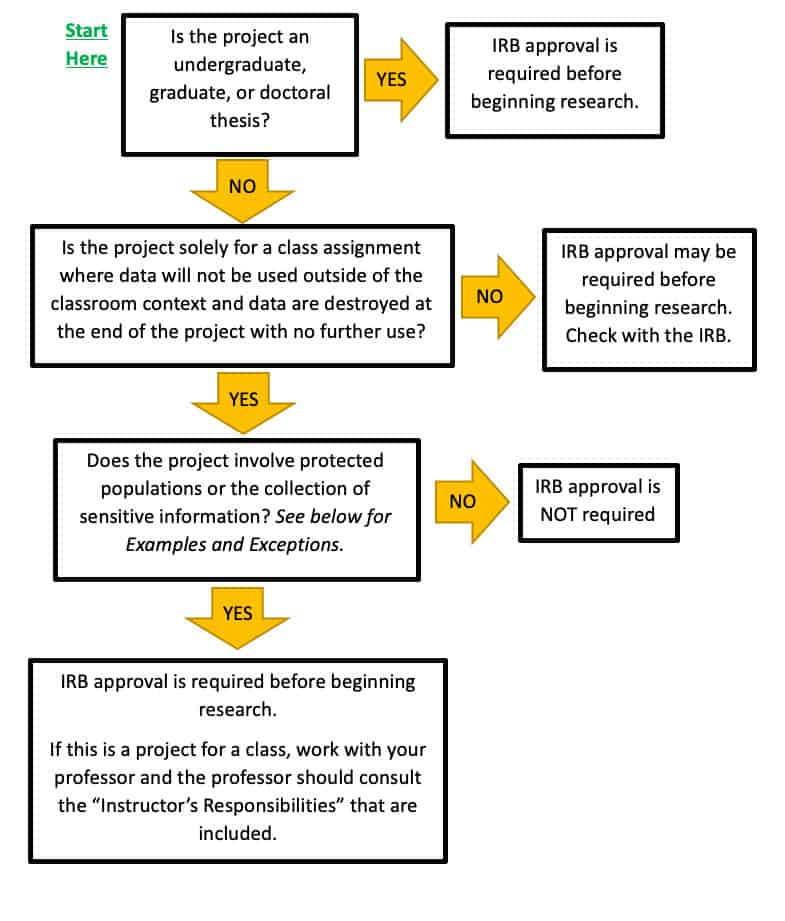
Federal regulations and university policies require approval of the Institutional Review Board (IRB) for research with human subjects. This applies whether research is conducted by faculty or students. However, some class projects are conducted for purely educational purposes and not as research, and thus, do not require IRB approval. Follow the flow chart below to determine if your project needs IRB approval.
NOTE: The IRB will not approve research retroactively, therefore, applications for IRB approval must be submitted and approved BEFORE any research is conducted.
How do I know if I must fill out an IRB Application?

Checklist for IRB application
- Answer all questions on the IRB application thoroughly.
- Include specific plans and forms for informed consent from all participants and if minors are involved in your study, parental consent must be included.
- Explain how you will keep participants anonymous throughout the study.
- Discuss your plan for keeping the data secure and deleting, destroying data at the conclusion of your study.
- Include specific survey questions, interview question, etc. and be sure they do not violate the rights or safety of your participants.
Protected Populations
Examples include, but are not limited to:
- Children/Minors (under the age of 18)
- Exception – projects conducted in established or commonly accepted educational settings involving normal educational practices.
- Prisoners
- Pregnant women
- People with diminished capacity to give consent
- Mentally or physically challenged individuals
Sensitive Information
Examples include, but are not limited to:
- Information relating to an individual’s psychological wellbeing or mental health
- Information relating to sexual attitudes, preferences, or practices
- Information relating to the use of alcohol or drugs
- Information relating to illegal behavior
- Information that if released could reasonably place the individual at risk of criminal or civil liability or be damaging to the individual’s financial standing, employability, or reputation
- Information that would normally be recorded in a patient’s medical record and the disclosure could reasonably lead to discrimination, stigmatization, etc.
Instructors’ Responsibilities
If the study is required for a class project:
Instructors should meet with students as soon as possible and go over these guidelines and use the flow chart on this webpage to determine if the proposed class project could be considered research. If there is any question and even a remote possibility that a class project may fall under the definition of research, instructors are advised to check with the chairperson of the IRB to discuss the study. Remember, class projects that involve protected populations or sensitive information (as defined in this document) require IRB review and approval.
The Process: The student (with guidance from the Instructor) must determine if IRB approval is needed (see process above). If IRB approval is needed, the student must submit a completed IRB application to the Instructor. The Instructor will work with the student until the application is completed in a satisfactory manner. The Instructor will then collect all of the IRB applications for that class and send them together to the chairperson of the IRB along with confirmation that the Instructor has reviewed the applications and approves of the quality and content of the applications.
If the Study does NOT require IRB approval, Instructors and students should read carefully through the Informed Consent Checklist (below) to be sure to consider these items in the design and implementation of the class project.
Informed Consent Checklist for studies that DO NOT require IRB approval.
- When information is collected for a class project that does not meet the definition of research (as defined by the federal regulations) and does not require approval by the IRB, it is still important to “inform” the participants about the class project, whether posted as an email message or presented verbally in person. Make sure the participant is told:
- The identity of the student collecting data. (This may not always be necessary, as many students enlist the help of friends or family to collect data for a class project)
- They must be at least 18 years of age to participate.
- It is a class project. (Give a little information, e.g., This is for my business class, I am trying to see if there is a relationship between X and Y.)
- What they will be asked to do (“I would like to ask you some questions about why you chose to attend this University. If there are any questions you don’t want to answer, it is fine to skip them.”).
- How long the interview, survey, etc., may take to complete.
- What will happen to the information collected (“The information will be used to write my paper for the class, and I will give a presentation in class. All of my notes, surveys, etc., will be destroyed when the project is completed.”)
- If they will be identified: examples: “I will not write your name on my notes”; “Do not write your name on the survey;” “I will not use your name in my paper.”
- The student’s and instructor’s contact information if they have any questions (provide phone number/email).
*The IRB at Cairn University acknowledges the consultation and adaptation of content of IRB guidelines from the websites of the University of Maine, University of North Carolina, Midwestern State University, TX, and Saint Louis University.
